DYANAVEL® XR delivers
Consistent delivery, regardless of formulation1
Eligible patients may PAY $25
Learn more about Savings & SupportUnique technology allows you to adjust DYANAVEL® XR (amphetamine) tablet to the patient’s needs without breaking down the delivery system1

- Scored 5-mg tablet allows dosing up or down in small increments
- Dosing as low as 2.5 mg to help balance side effects and efficacy
- Tablets must be taken in the morning, with or without food

Up to 8 Dosing Options—the Most of Any Extended-Release Amphetamine Tablet
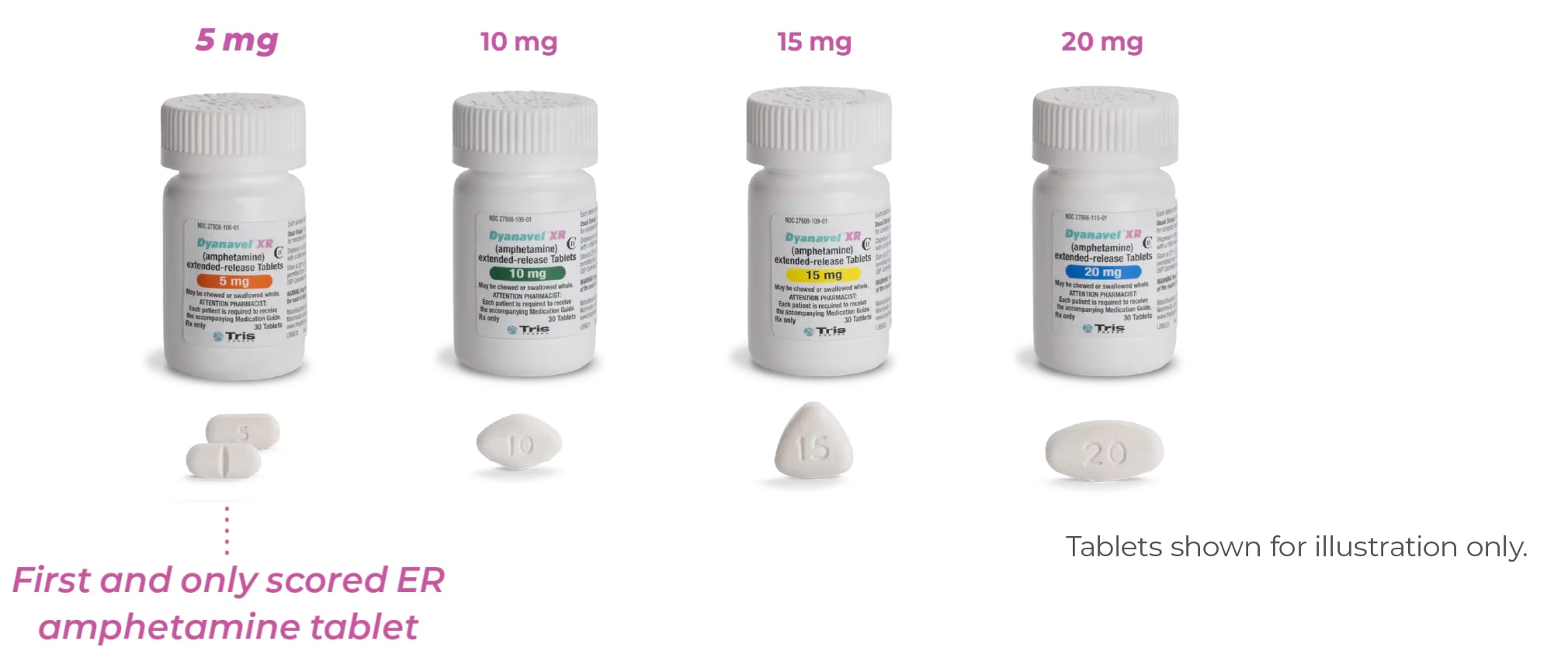
- The recommended starting dose is 2.5 mg or 5 mg once daily in the morning
- Adjust dose in 2.5-mg to 10-mg increments per day, every 4 to 7 days, until optimal response is achieved or maximum dose is reached
- The maximum recommended dosage is 20 mg once daily
If switching from other amphetamine products to DYANAVEL XR:
- Discontinue other amphetamine treatment, then titrate with DYANAVEL XR following the above titration schedule
- Do not substitute for other amphetamine products on a milligram-per-milligram basis because of different amphetamine base compositions and differing pharmacokinetic profiles
Prior to treatment with DYANAVEL XR:
- Assess for the presence of cardiac disease (ie, perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) and risk for abuse
- After prescribing, keep prescription records, educate patients about abuse, monitor for abuse and overdose, and reevaluate the need for DYANAVEL XR use
- Periodically reevaluate the long-term use of DYANAVEL XR, and adjust dose as needed
ADHD, Attention Deficit Hyperactivity Disorder; ER, extended-release.
References: 1. Dyanavel XR [package insert]. Tris Pharma, Inc., Monmouth Junction, NJ. 2. Herman BK, King TR, Kando JC, Pardo A. Poster presented at: Neuroscience Education Institute Congress; November 7-11, 2018; Orlando, FL.


